Recently, Professor Cao Hongzhi's group published a researcharticle entitled "Redox-ControlledSite-Specific α2‒6-Sialylation" on Journal of the American Chemical Society (IF: 14.357). Professor Cao from SDU isthe corresponding author of this paper. Researchers from the University of California, San Diego, Nankai University and Zhejiang University also participated in the work.
Sialic acid is a monosaccharide with a nine-carbon backbone. It is an important component of common glycoproteins and glycolipids in human and other mammals. Sialic acid-containing glycans play vital roles in many physiological and pathological processes. Sialylation of glycans is catalyzed by various sialyltransferasesin vivo. Due to the limited source and strict substrate specificity of mammalian sialyltransferases, many complexsialoglycans have not been enzymatically synthesizedin vitroyet.
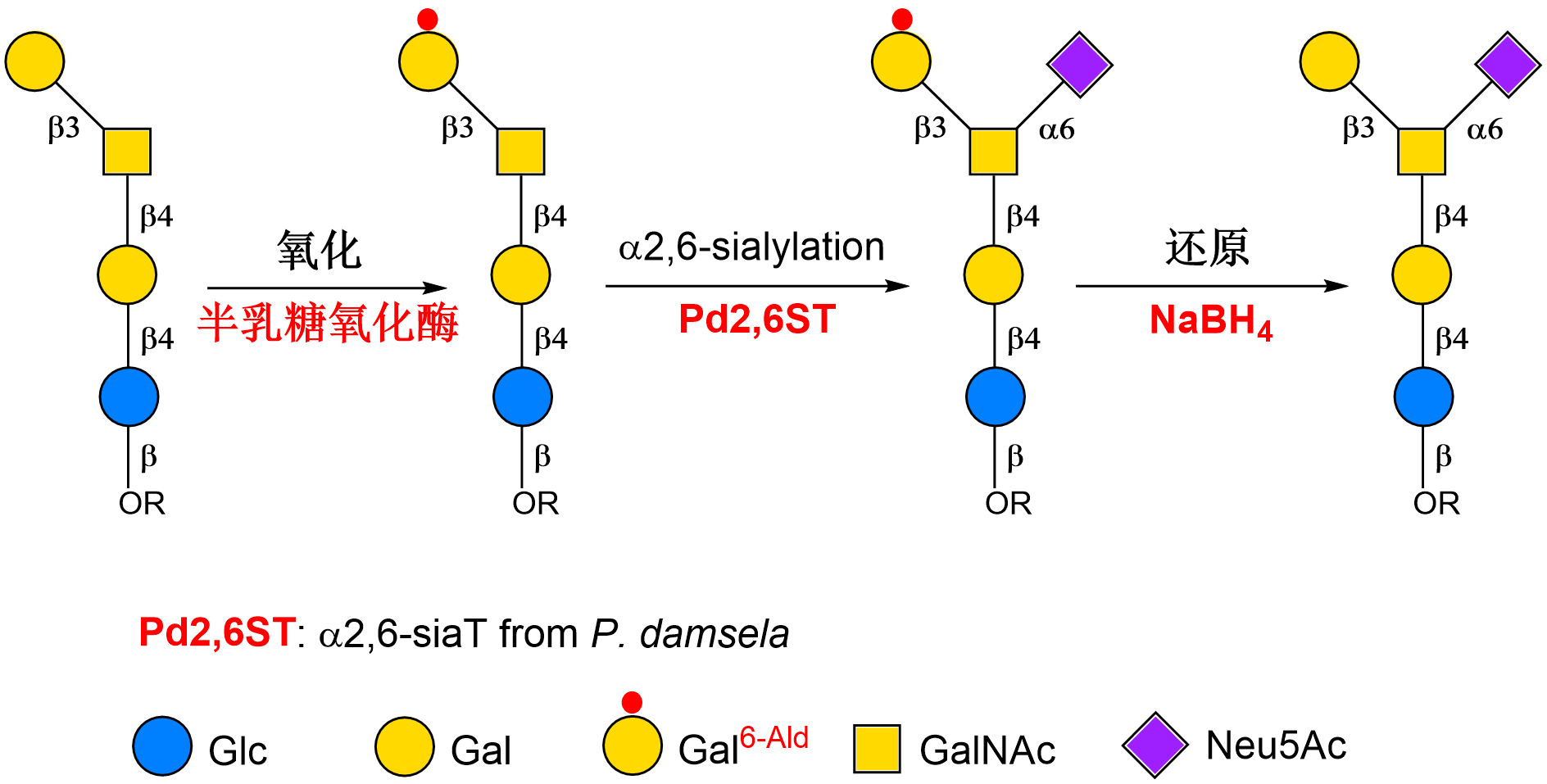
Photobacterium damselaeα2–6sialyltransferase(Pd2,6ST) is the first discovered bacterialα2–6sialyltransferase, which has been widely used in the construction of various sialylated glycans due to its high expression level, excellent substrate flexibility and remarkable activity. However, Pd2,6ST has poor regioselectivity and cannot realize site-specificα2–6sialylation on complex substrates containing multiple sialylation sites. In order to solve this problem, Cao’s group innovatively developed a "substrate engineering" strategy in an earlier study, which achieved the regioselectiveα2–6sialylation of some specific complex glycans by controlling the conformation of receptor substrates (J. Am. Chem. Soc.2014, 136, 5205).
Based on the previous work, a general redox-controlled site-specific sialylation strategy using Pd2,6ST was developed. This strategy features site-specific enzymatic oxidation of galactose units to mask the unwanted sialylation sites and precisely controlling the site-specific α2−6-sialylation at intact galactose orN-acetylgalactosamine units. With multiple enzyme synthesis modules, a series of complex sialylated glycans with important biological activities are rapidly and efficiently synthesized from simple monosaccharides. Furthermore, the interaction and structure-activity relationship between sialylated glycans and their receptor molecules are systematically studied using glycan array technology, which is of great significance to explore the molecular mechanism of sialylated glycans in the processes of inflammation and pathogen invasion.
Cao Hongzhi, thecorrespondingauthor of this paper, is currently a professor of the National Glycoengineering Research Center. The research of his team focuses on the development of efficient and transferable methods for the systematic synthesis of complex human glycan libraries. Using more than 20 delicately designed enzymatic assembly modules, Cao’sgroup has successfully constructeda series of complex human glycan libraries. Related research workhas been published in internationally renowned journals such asJ. Am. Chem. Soc.,Angew. Chem. Int. Ed.,ACS Catal.This work is under the support of the National Natural Science Foundation of China, open project of the State Key Laboratory of Microbial Technology of Shandong University and the "Innovative Team of Pharmaceutical Chemical Biology of Shandong University".
Related reading:
https://pubs.acs.org.ccindex.cn/doi/pdfplus/10.1021/jacs.9b00044
Website of Cao’s group:
https://caolab.wixsite.com/hongzhicao
Written by: Lu Na and Liu Changcheng
Edited by: Che Huiqing