Recently, Prof. Xu Zhenghu’s Research Group from the School of Chemistry and Chemical Engineering, have made breakthroughs in the research of controllable and selective synthesis of complex polycyclic compounds. The results were published in the internationally renowned journal of chemistry《Angew. Chem.》with the title of " Asymmetric Synthesis of a Fused tricyclic Hydronaphthofuran Scaffold by Desymmetric [2+2+2] Cycloaddition". Teng Qi is the first author of this paper, Prof. Xu Zhenghu is the corresponding author, and the School of Chemistry and Chemical Engineering of Shandong University is the first communication unit.
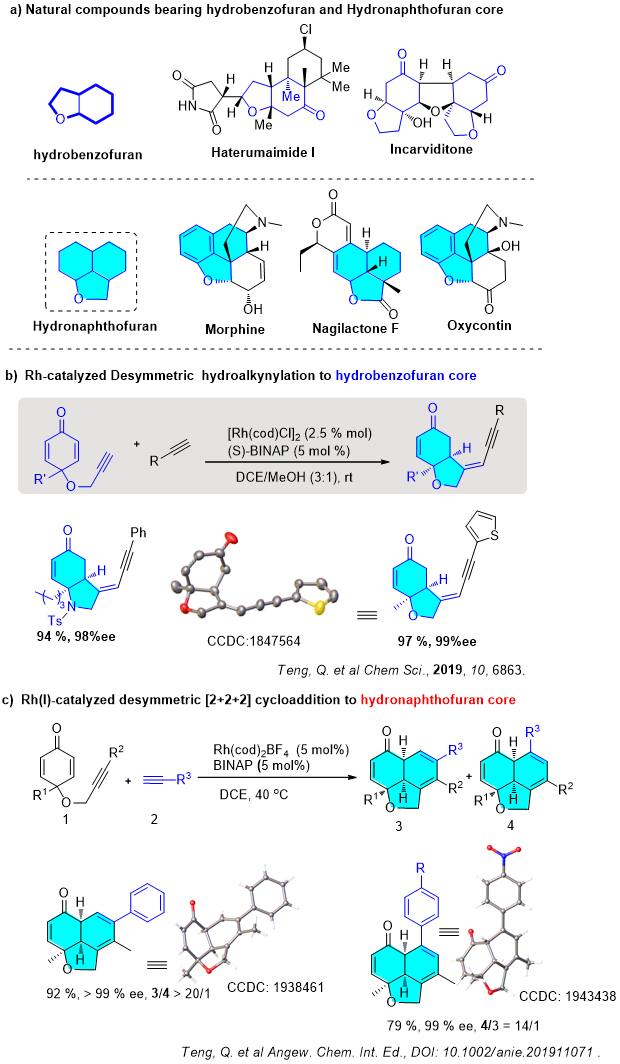
Many biologically active natural products and drug molecules contain complex polycyclic ring systems (Figure a), typical chiral bicyclic structures such as cis-hydrobenzofuran, and chiral tricyclic structures such as hydronaphthofuran scaffold. The well-known morphine and painkiller Oxycontin contain similar complex tricyclic systems. Traditional methods for synthesizing these bicyclic / tricyclic backbones usually requires multiple steps, which is relatively inefficient, and the establishment of multiple chiral centers in these multiple steps also poses greater challenges. Therefore, how to achieve a highly selective and controllable synthesis of such a dense bicyclic / tricyclic skeleton in one step through asymmetric catalytic reactions has always been a challenge for synthetic chemists.
Very recently, Xu's group achieved the tandem addition cyclization of1,6-enyneswith terminal alkynes to efficiently build a bicyclic hydrogenated benzofuran product by rhodium-catalyzed desymmetric reaction (Figure b). The selective dimerization of two terminal alkynes can theoretically produce 12 isomers. One product was produced from tens of possible isomers through precise control of chemo-, regio-, and stereoselectivities using a single rhodium catalyst (Chem. Sci.2019,10, 6863).
On the basis of this previous results, Xu’s group adjusted the structure of the 1,6-enyne and further optimized the reaction conditions to construct the diverse fused tricyclic hydronaphthofuran scaffolds with three consecutive stereogenic centers in one step from easily available materials with excellent chemo-, regio-, diastereo-, and enantioselectivities (Figure c). Notable features of these reactions include 100% atom-economy, very broad scope, and mild reaction conditions.
The link of this paper:https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201911071
Written by: Teng Qi
Edited by:Che Huiqing