Recently, Professor Gao Chengjiang’ team in the School of Basic Medical Sciences of Shandong University published a new paper entitled "Methyltransferase-Like Protein 14 Attenuates Mitochondrial Antiviral Signaling Protein Expression to Negatively Regulate Antiviral Immunity via N6-methyladenosine Modification" on Advanced Science (IF:15.84). PhD student Qin Fei is the first author of the paper, Professor Gao Chengjiang and assistant researcher Liu Bingyu of Shandong University are the co-corresponding authors of the paper, and Shandong University is the first author institute and the sole corresponding author institute. This research is supported by Professor Yi Fan and Professor Ma Chunhong.
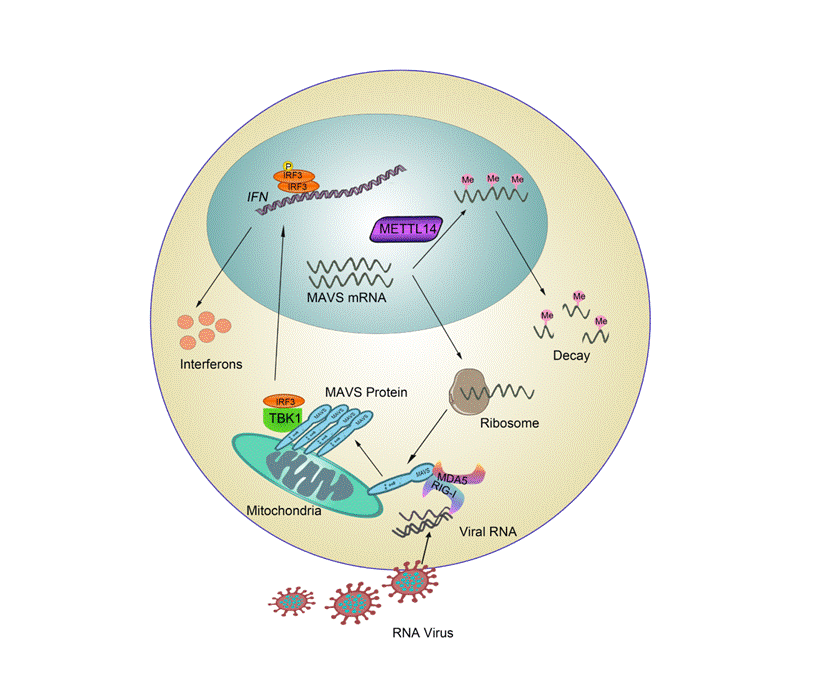
Intracellular viral RNA is detected by the retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), including retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5). The two receptors in the natural immune cells recognize the viral RNA released into the cytoplasm after infecting with a virus, and then transmit the infection signal to the key adaptor molecule MAVS. Activated MAVS rapidly forms very large aggregates on the mitochondrial membrane upon viral infection, activating the cytosolic kinases IKK and serine/threonine-protein kinase (TBK1) and interferon regulatory factor 3 (IRF3). IRF3 transfers into the nucleus, and then triggers the production of type I IFNs and other antiviral molecules. Among them, MAVS is the key linker molecule in this signaling pathway, and the study of its activation regulation mechanism has always been a hot focus in the research of innate immunity.
The previous research of this group found that the E3 ubiquitin ligase TRIM31 increased the ubiquitination of MAVS protein at position K63 and promoted the formation and activation of its prion-like aggregates (Nature Immunology, 2017). Then they found that deubiquitinating enzymes can recruit TRIM31 to the mitochondria to regulate the ubiquitination modification and aggregation activation of MAVS independently of its enzymatic activity (Nature Communications, 2021). However, the molecular regulatory mechanism of post-transcriptional modification on MAVS mRNA remains indistinct. Methylation of adenosine at the N6position (m6A) of RNA has been identified as the most common mammalian mRNA modification, which can modulate enormous genes expression and regulate extensive biological activities including metabolism, tumor progression, circadian clock, and DNA damage response. The article recently published by Professor Gao Chengjiang’s team in Advanced Science shows that METTL14, a key component of m6A methyltransferase, can regulate the stability of MAVS mRNA to negatively regulate the production of type I interferon and the innate antiviral response. Compared to wild-type mice, heterozygotesMettl14deficientmice better tolerate RNA virus infection. The m6A modification at 131,246,518 sites on Mavs mRNA by MTTL14 impaired its mRNA stability and the production of protein, which inhibited the antivirus innate immunology response. This study revealed a new mechanism for regulating the stability of MAVS transcripts through m6A modification, and further improved the regulation mechanism of antiviral innate immune signal pathway.
Professor Gao Chengjiang’s team has been dedicated to the study of the regulation mechanism of innate immune signal transduction for a long time, systematically exploring the regulation mechanism of anti-viral innate immune signal transduction and inflammatory response. A number of research articles have been published onNature Immunology,Advanced Science, Journal of Experimental Medicine,Nature Communications,Cell Death & Differentiation,PLOS Pathogens,Journal of Immunologyand other international authoritative journals. This work was supported by the Innovative Research Group Project of Shandong University. This work was also supported by the Natural Science Foundation of China and the Postdoctoral Science Foundation of China.
Link to this paper:
https://onlinelibrary.wiley.com/doi/10.1002/advs.202100606