Recently, the joint research group of Prof. Zhang Wei, Li Shengying and Bian Xiaoying from the State Key Laboratory of Microbial Technology discovered a novel enzymatic sulfur-incorporation mechanism in the biosynthetic pathway of chuangxinmycin. The results were published as a Very Important Paper (VIP) on Angewandte Chemie International Edition entitled "Biosynthesis of chuangxinmycin featuring a deubiquitinase-like sulfurtransferase".Prof. Zhang Wei is the primary corresponding author, Prof. Li Shengying and Bian Xiaoying are the co-corresponding authors. Assistant professor Zhang Xingwang is the first author. Shandong University is the first and corresponding affiliate.
Sulfur is an essential element for all life by forming various sulfur-containing molecules.The most intriguing part of sulfur-containing compound biosynthesis is the mechanism of how the sulfur atom is incorporated, which is normally "hijacked" from primary metabolism or derived from sulfides. New chemistry and unusual enzymatic mechanisms have constantly been discovered from the "S" communications not only between primary and secondary metabolisms, but also between inorganic and organic worlds. Thus, studies on enzymatic sulfur-incorporation mechanism are of special interest both for biologists and chemists.
Chuangxinmycin (1),originally isolated from Actinoplanes tsinanensis in 1970s by Chinese scientists,is a sulfur-containing antibiotic with a unique thiopyrano[4,3,2-cd]indole (TPI) skeleton. Chuangxinmycin showed selective inhibitory activity against bacterial tryptophanyl-tRNA synthetase,although its clinical application has been suspended due to the narrow antimicrobial spectrum, it still draws continuing attention on drug development since there has not been any clinical antibiotic targeting bacterial TrpRS.
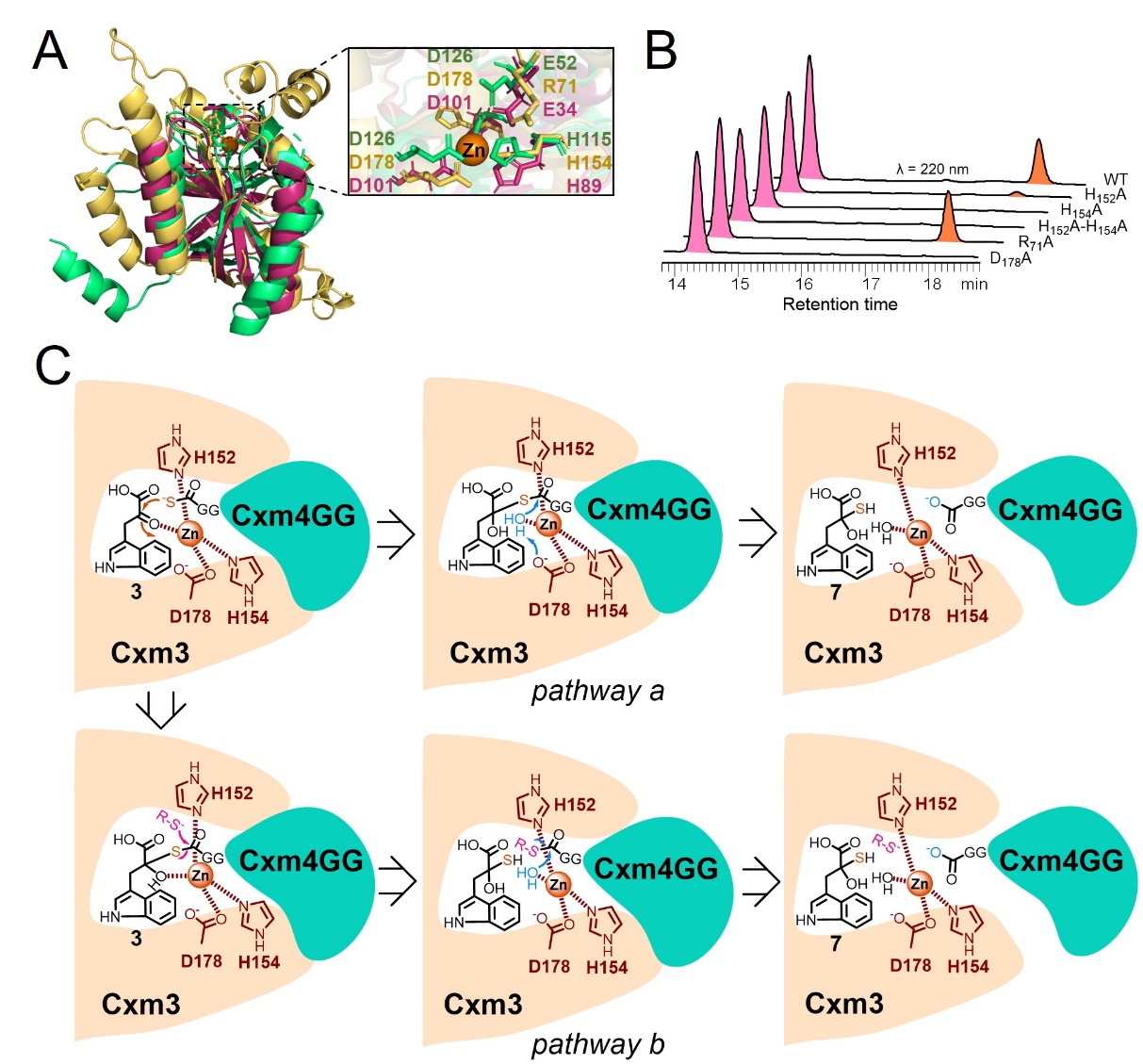
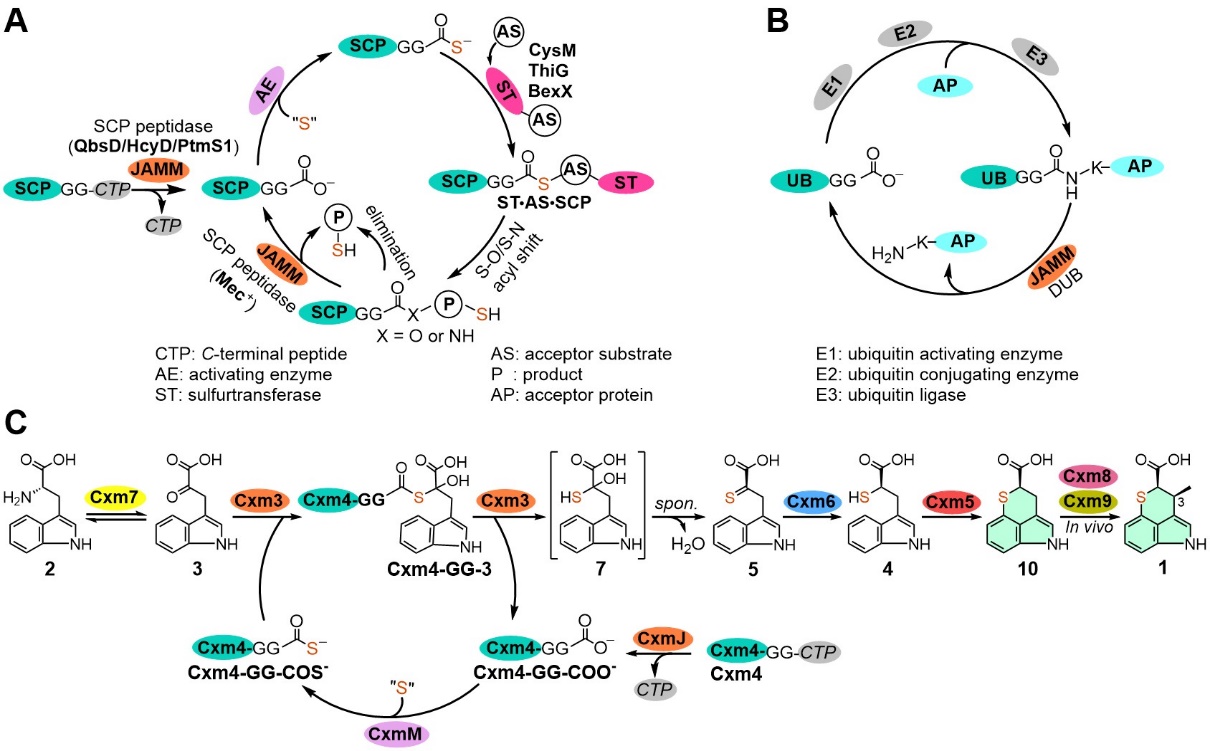
In this study, by using a combined method of bioinformatic analysis, enzymatic function reconstitution and product identification, the JAMM/MPN+ familymetallopeptidase Cxm3 was firstly revealed to catalyze the unprecedented sulfur-transfer reaction from the activated sulfur carrier protein (Cxm4GG-COS-) to the substrate. To further understand the catalytic mechanism, artificial intelligence (AI) dependent protein folding and key catalyticresidue mutation experiments were carried out. As a result, the deubiquitinase-likeCxm3 was found to mediate an atypical sulfur-transfer reaction by interacting with the ubiquitin-like Cxm4GG, in which a novel Zn2+dependent C-S bond formation mechanism was involved.
Based on the established sulfur-transfer reaction, the authors also elucidated the whole enzymatic steps involved in the TPI skeleton biosynthesis. Finally, the de novo biosynthesis of 3-demethylchuangxinmycin was reconstituted in vitro by a "one-pot" reaction from the startingmaterialL-tryptophan.
In conclusion, this study elucidated and reconstituted the biosynthetic pathway of the unique TPI skeleton in 1 by in vitro enzymatic approaches. Particularly, a novel JAMM/MPN+ superfamily enzyme Cxm3 was characterized as a Zn2+-dependent DUB-like sulfurtransferase, which broadens the chemistry of JAMM/MPN+ proteins. Conserved domain and cladogram analysis revealed that Cxm3 and its uncharacterized homologues from various actinomycetes genera form a new clade separated from other subfamilies. Accordingly, it is envisioned that the co-occurring counterparts of DUB-like Cxm3 and UB-like Cxm4 may have evolved independently for secondary metabolism in prokaryotes, apart from the UB signaling functions in eukaryotes and archaea.
Resolution of these biosynthetic mysteries in Chuangxinmycin highlights the intriguing strategies for creating sulfur-containing molecules in nature. Our work also sets the molecular basis for enzymatically generating TPI derivatives as potential therapeutic agents.
This work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and other fund projects.